Activated Carbon: What Is Activated Carbon & How It Works
Unpack what is activated carbon. This guide explains its function in filtration, production, key properties, and why it's crucial for water & gas purification, including biogas.
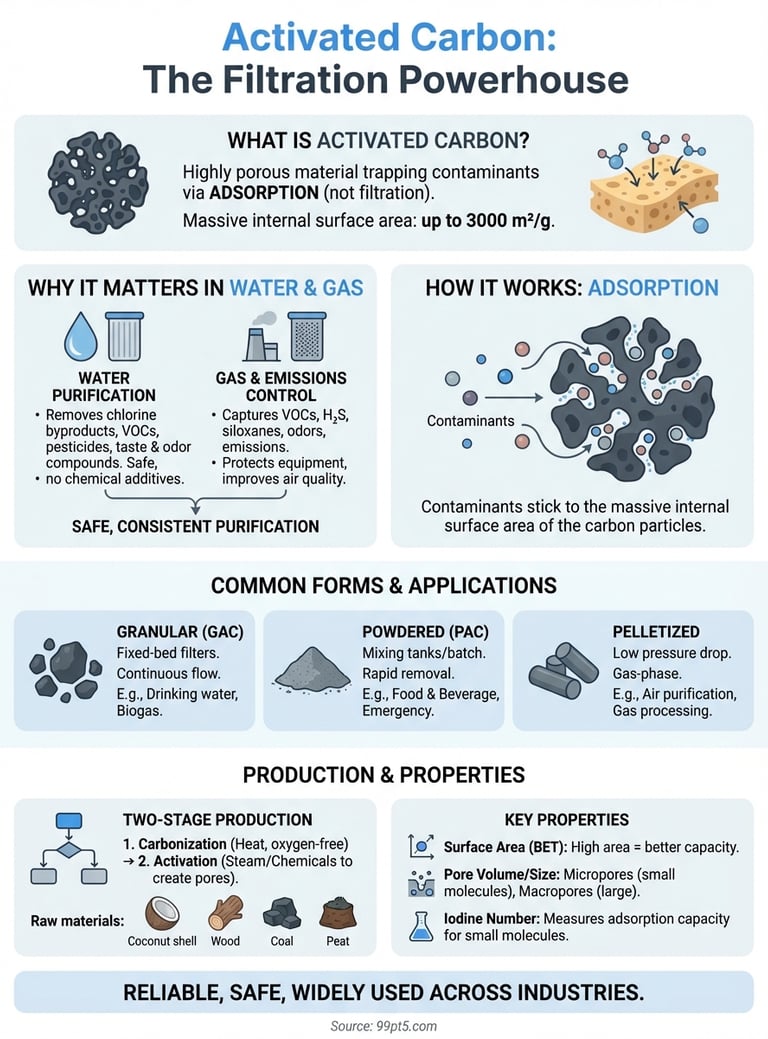
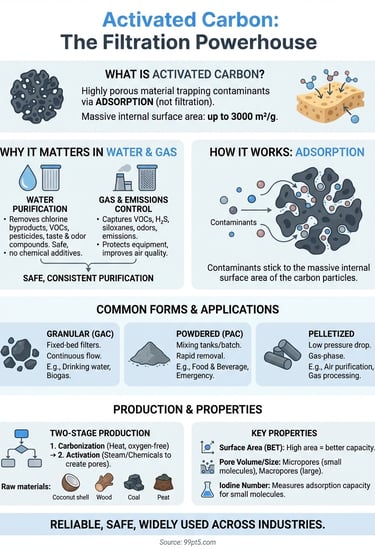
Activated carbon is a highly porous material that traps contaminants from gases and liquids. You find it in water filters, air purification systems, and industrial gas processing equipment where it captures organic compounds, odors, and impurities through a process called adsorption. The material works because its surface area is massive. One gram of activated carbon can have up to 3000 square meters of internal surface area. That gives it exceptional filtering power in a compact form.
This article breaks down what activated carbon is and how it works in filtration systems. You'll learn why this material matters in gas and water treatment, how to use it effectively in filtration setups, what raw materials create it, and the key properties that make different types suitable for specific applications. We'll also cover where activated carbon gets used in practice, from drinking water purification to biogas processing. By the end, you'll understand why this material remains one of the most reliable and widely used purification methods across industries.
Why activated carbon matters in gas and water
Your water and gas purification systems depend on activated carbon because it removes contaminants that other methods miss or handle inefficiently. Traditional filtration catches particles, but activated carbon targets dissolved organic compounds, chlorine, volatile organic compounds (VOCs), and odor-causing molecules at the molecular level. Industries rely on this material because it delivers consistent purification without requiring chemical additives or generating harmful byproducts. When you compare activated carbon to alternatives like chemical treatment or membrane filtration, you'll find it offers a simpler, safer, and often more cost-effective solution for removing specific types of contaminants.
Impact on water quality and safety
You need activated carbon in water treatment because it removes chlorine byproducts, pesticides, pharmaceutical residues, and taste or odor compounds that make water undrinkable or unsafe. Municipal water systems use granular activated carbon filters to meet drinking water standards and eliminate disinfection byproducts like trihalomethanes. Industrial facilities rely on it to treat process water, remove organic solvents, and prepare wastewater for discharge or reuse. The material works without altering the water's pH or mineral content, which means you get purified water without the complications that come with chemical treatment methods.
Activated carbon removes contaminants without adding chemicals to your water, making it one of the safest purification methods available.
Role in gas purification and emissions control
Gas processing systems use activated carbon to capture VOCs, hydrogen sulfide, mercaptans, and other organic compounds that cause odors, toxicity, or equipment corrosion. Biogas upgrading systems depend on activated carbon to remove siloxanes, hydrogen sulfide, and moisture before the gas enters compression or combustion equipment. Air purification units in industrial facilities use it to control emissions and protect workers from harmful vapors. The material handles fluctuating gas compositions and flow rates better than many alternatives, which makes it reliable in applications where gas quality varies throughout the day or season.
How to use activated carbon in filtration systems
You install activated carbon in filtration systems by choosing the right physical form, sizing your vessel correctly, and maintaining proper flow rates for effective contaminant removal. The success of your filtration system depends on matching the carbon type to your specific application and operating it within the manufacturer's recommended parameters. Understanding what is activated carbon and how different forms behave in filters lets you design systems that deliver consistent performance while minimizing operating costs and maintenance requirements.
Selecting the right carbon form for your application
Your choice between powdered, granular, or pelletized activated carbon determines how you'll integrate it into your system. Granular activated carbon (GAC) works best in fixed-bed filters where water or gas flows through a stationary carbon bed, making it ideal for continuous operations like drinking water treatment or biogas purification. Powdered activated carbon (PAC) requires mixing tanks and filtration equipment to separate it from the treated liquid, which suits batch processes or applications where you need rapid contaminant removal. Pelletized carbon offers lower pressure drop and works well in gas-phase applications where air or process gases flow upward through the bed.
Particle size affects both performance and pressure drop across your filter. Smaller particles provide faster adsorption because contaminants reach the carbon's internal pores more quickly, but they also create higher resistance to flow. Larger particles reduce pumping costs but require longer contact times to achieve the same purification level. You balance these factors based on your flow rates, available pressure, and required treatment efficiency.
Installing carbon in filter vessels
Your filter vessel design determines how effectively activated carbon contacts the fluid stream. Downflow configurations work for most water applications, where liquid enters at the top of the vessel and flows through the carbon bed by gravity or pressure. Upflow designs prevent channeling in gas applications and keep the carbon bed expanded, which reduces pressure drop and ensures uniform gas distribution across the entire bed. You need adequate headspace above the carbon bed to prevent material loss during backwashing or when gas flow rates fluctuate.
Contact time drives your vessel sizing calculations. You calculate the empty bed contact time (EBCT) by dividing the bed volume by your flow rate, with typical values ranging from 5 to 15 minutes for water treatment and 2 to 10 seconds for gas purification. Deeper beds provide longer contact times and better contaminant removal, but they also increase your initial carbon costs and system footprint.
Proper vessel sizing ensures your activated carbon has enough contact time with the fluid stream to capture target contaminants effectively.
Operating and monitoring carbon filters
You maintain filter performance by monitoring pressure drop, breakthrough indicators, and flow rates throughout the carbon's service life. Pressure drop across the bed increases as the carbon captures contaminants and particles, signaling when you need backwashing or carbon replacement. Regular sampling at the filter outlet tells you when contaminants start breaking through, which indicates the carbon is approaching exhaustion. You track these parameters daily in critical applications like drinking water treatment or weekly in less demanding systems.
Backwashing extends carbon life in water applications by removing trapped particles and redistributing the bed. You backwash by reversing the flow direction at rates high enough to expand the bed by 30 to 50 percent, which releases trapped solids without washing carbon out of the vessel. Most systems require backwashing every few days to several weeks depending on the feedwater quality and the types of contaminants you're removing.
What activated carbon is made of and produced
You create activated carbon from carbon-rich organic materials through a two-stage thermal process that develops its porous structure and massive surface area. The production starts with raw materials like coconut shells, wood, coal, peat, or agricultural waste that contain high carbon content and minimal inorganic impurities. Manufacturers select specific raw materials based on the desired pore structure and application requirements, since each source produces carbon with distinct physical and chemical characteristics. Understanding what is activated carbon and how different source materials affect its properties helps you choose the right product for your filtration needs.
Raw materials that create activated carbon
Your activated carbon's performance depends heavily on which raw material the manufacturer uses during production. Coconut shells produce carbon with predominantly small pores (micropores less than 2 nanometers), making them excellent for capturing small organic molecules from water or gases. Wood-based carbons develop larger pores (macropores greater than 50 nanometers) that work better for removing color compounds, proteins, and larger organic molecules from liquids like vegetable oils, alcoholic beverages, or sugar solutions.
Bituminous coal creates carbon with a balanced pore distribution across all size ranges, which makes it versatile for applications requiring removal of both small and large contaminants. Lignite and peat produce softer carbons that work well in powder form but break down too easily for fixed-bed applications. Agricultural byproducts like rice husks, fruit pits, and bamboo offer sustainable alternatives that match or exceed the performance of traditional materials in specific applications.
The raw material you select determines the pore size distribution in your activated carbon, which directly affects which contaminants it can capture most effectively.
The two-stage production process
Your manufacturer produces activated carbon through carbonization followed by activation, with each stage serving a specific purpose. Carbonization involves heating the raw material to 400 to 600 degrees Celsius in an oxygen-free environment, which drives off volatile compounds and leaves behind a carbon-rich char with limited porosity. This char has minimal adsorption capacity until it undergoes activation to develop the extensive internal pore structure.
Physical activation exposes the carbonized material to oxidizing gases like steam or carbon dioxide at temperatures between 800 and 1100 degrees Celsius. These gases react with carbon atoms to create pores and increase surface area, producing activated carbon with up to 3000 square meters of surface area per gram. You control the pore size distribution by adjusting temperature, time, and gas composition during this activation stage.
Chemical activation combines carbonization and activation into a single step by impregnating the raw material with dehydrating chemicals like phosphoric acid, potassium hydroxide, or zinc chloride before heating. This method operates at lower temperatures (450 to 700 degrees Celsius) and produces carbon with higher yields and better-developed pore structures. Manufacturers must wash the finished carbon thoroughly to remove residual chemicals before you can use it in water or gas purification applications.
Key properties and types of activated carbon
You evaluate activated carbon quality through measurable properties that predict how it will perform in your specific application. Surface area, pore volume, particle density, and adsorption capacity determine which carbon works best for removing target contaminants from your water or gas streams. These properties vary significantly between different carbon types and source materials, which means you need to match the specifications to your filtration requirements rather than assuming all activated carbons perform identically. When you ask what is activated carbon and how to select it, these key properties provide the technical criteria for making informed purchasing decisions.
Surface area and pore volume measurements
Your activated carbon's effectiveness depends on its total surface area and how that area distributes across different pore sizes. Manufacturers measure surface area using the BET method (Brunauer-Emmett-Teller), which typically yields values between 500 and 3000 square meters per gram. Higher surface areas generally indicate better adsorption capacity, but you also need to consider pore size distribution since different contaminants require different pore dimensions to enter and adsorb effectively.
Pore volume tells you how much space exists within the carbon structure for capturing contaminants. Micropores (less than 2 nanometers) provide most of the surface area and capture small molecules. Mesopores (2 to 50 nanometers) serve as transport pathways that let contaminants reach the micropores. Macropores (greater than 50 nanometers) handle large organic molecules and prevent pore blockage in applications with high contaminant loading.
Iodine number and adsorption capacity
You use the iodine number as a standard indicator of activated carbon's adsorption capacity for small molecules. This test measures how many milligrams of iodine one gram of carbon adsorbs from solution, with typical values ranging from 500 to 1200 milligrams per gram. Higher iodine numbers indicate better micropore development and superior performance for capturing small organic compounds, VOCs, and dissolved contaminants from water.
Different tests measure adsorption capacity for specific applications. The methylene blue number indicates how well carbon removes color compounds and larger molecules. The molasses number predicts performance in decolorizing sugar solutions and other liquids with high molecular weight impurities. You review these test results alongside your contaminant profile to select carbon that targets your specific purification needs.
The iodine number gives you a reliable benchmark for comparing different activated carbons, but you should also consider application-specific tests for the most accurate performance prediction.
Common types by physical form
Granular activated carbon comes in irregular particles sized between 0.2 and 5 millimeters that you pack into fixed-bed filters. This form offers the best balance of adsorption capacity, pressure drop, and ease of handling for most water and gas treatment applications. You can reactivate and reuse granular carbon multiple times, which reduces your long-term operating costs compared to single-use alternatives.
Powdered activated carbon consists of fine particles less than 0.18 millimeters (80 mesh) that you dose directly into treatment tanks. Fast kinetics make this form ideal for emergency treatment, seasonal taste and odor control, or batch processing applications. Pelletized carbon shapes the material into cylindrical extrudates between 0.8 and 5 millimeters in diameter that create minimal pressure drop in gas-phase applications requiring high flow rates.
Where activated carbon is used in practice
You find activated carbon in diverse applications across multiple industries because it removes contaminants safely, efficiently, and without producing harmful byproducts. Municipal water systems, industrial facilities, food processors, and environmental control operations rely on this material to meet quality standards, protect equipment, and comply with environmental regulations. Understanding what is activated carbon and where it gets applied helps you recognize opportunities to improve your own purification processes or solve contamination problems that other methods handle poorly.
Municipal water treatment plants
Your drinking water passes through activated carbon filters that remove chlorine, taste and odor compounds, disinfection byproducts, and organic contaminants. Treatment plants use granular activated carbon in large concrete vessels where water flows downward through beds that range from 1 to 3 meters deep. These installations process millions of liters daily while meeting strict safety standards for public health protection. Municipal operators replace or reactivate the carbon every 1 to 5 years depending on the source water quality and contaminant loading rates.
Industrial gas processing and biogas upgrading
Gas purification systems depend on activated carbon to remove hydrogen sulfide, siloxanes, volatile organic compounds, and moisture before the gas enters engines, turbines, or distribution networks. Biogas facilities use it to upgrade raw biogas into pipeline-quality biomethane by removing contaminants that cause corrosion, odors, or equipment damage. The carbon protects downstream compressors and processing equipment from compounds that would otherwise reduce efficiency or create maintenance problems. Industrial operators typically arrange the carbon in vertical vessels where gas flows upward through pelletized or granular media at contact times between 2 and 10 seconds.
Activated carbon protects your gas processing equipment by removing corrosive and harmful compounds before they reach sensitive components.
Food and beverage production
Food processors use activated carbon to decolorize sugar solutions, purify vegetable oils, and remove unwanted flavors from alcoholic beverages. Sugar refineries apply powdered carbon to remove color-causing proteins that would make the final product dark or cause it to ferment prematurely. Distilleries and wineries use granular carbon to improve product clarity and remove off-flavors while preserving desirable taste characteristics. You also find it in water treatment systems that supply production lines requiring high-purity process water for mixing, washing, or product formulation.
Final thoughts
You now understand what is activated carbon and how it removes contaminants from water and gas streams through adsorption. This material delivers reliable purification across diverse applications because its massive surface area and porous structure trap organic compounds, odors, and impurities at the molecular level. Your choice of carbon type, physical form, and operating parameters directly determines how effectively your filtration system performs in removing target contaminants while minimizing operating costs and maintenance requirements.
Industries from municipal water treatment to biogas upgrading depend on activated carbon because it works safely without chemical additives or harmful byproducts. When you need advanced biogas purification systems that integrate activated carbon with other processing technologies, you'll find solutions designed to guarantee exceptional performance in gas upgrading. These systems maximize biogas recovery and reduce operational expenses while meeting environmental standards.