Top 7 H2S Removal Technologies: How They Work, Costs, Uses
This guide details 7 h2s removal technologies: how they work, costs, & ideal uses. Pick the perfect solution for your biogas or natural gas project.
Hydrogen sulfide tears through pipelines, poisons equipment, and creates safety hazards that can shut down your entire operation. When you're designing or specifying a biogas or gas processing system, getting H2S removal right means the difference between a profitable project and one that bleeds money through maintenance, downtime, and compliance issues. You need removal efficiency above 99%, predictable operating costs, and a technology that actually fits your feedstock quality and project scale.
This guide breaks down the seven most effective H2S removal technologies used in biogas and natural gas applications. You'll see exactly how each system works, what it costs to buy and operate, which gas compositions it handles best, and where each technology delivers the strongest performance. By the end, you'll know which approach suits your next project and what questions to ask vendors before you commit.
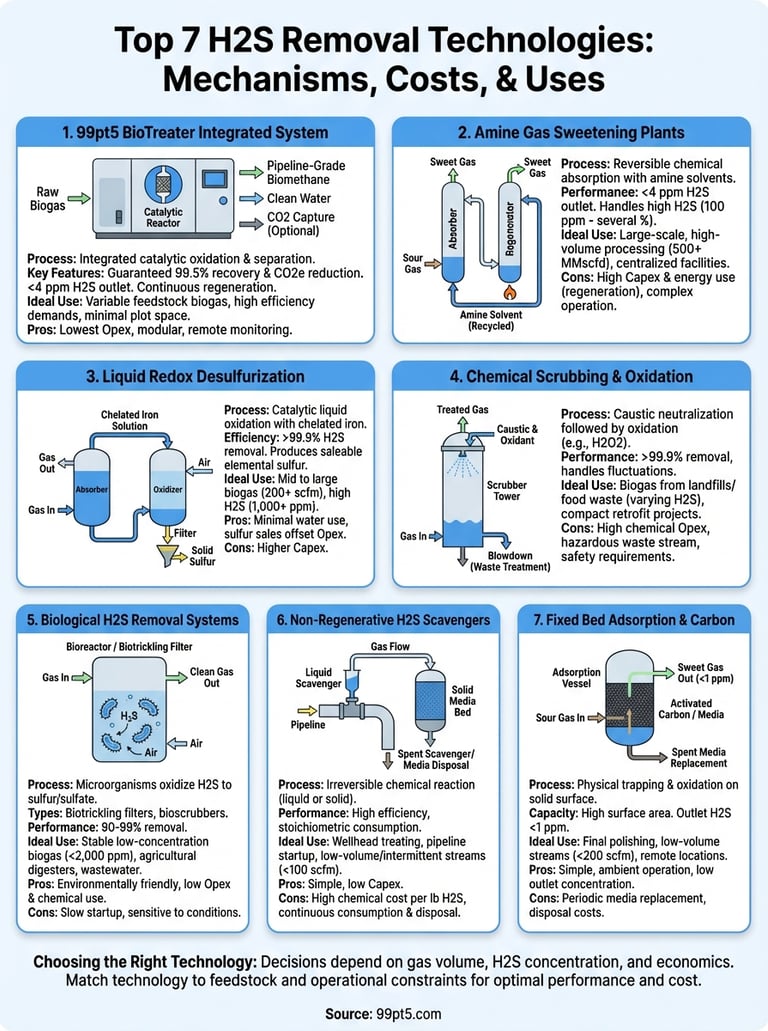
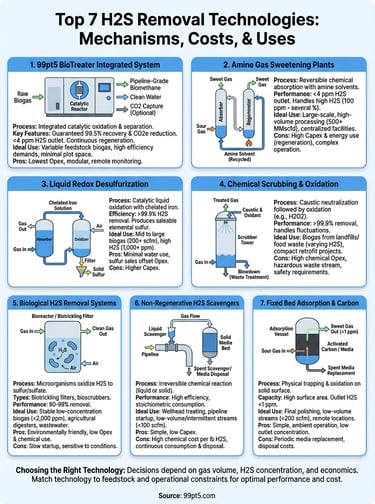
1. 99pt5 BioTreater integrated system
The BioTreater combines multiple H2S removal technologies into a single automated unit that takes raw biogas from your digester and delivers pipeline-grade biomethane in one continuous process. You get desulfurization, oxygen control, water removal, and optional CO2 capture without managing separate equipment packages or coordinating multiple vendors. This integrated approach cuts your installation time, reduces your plot space, and eliminates the interface problems that plague multi-vendor systems.
How the BioTreater removes H2S
The system uses a proprietary catalytic process that converts hydrogen sulfide into non-hazardous sulfur compounds through controlled oxygen injection. Unlike iron sponge or activated carbon beds that saturate and require disposal, this catalytic reactor continuously regenerates so you avoid the recurring costs and logistics of adsorbent replacement. The process operates at medium pressure, which lets you maintain efficiency while keeping compression costs reasonable.
Performance, guarantees, and specs
You receive a written guarantee of 99.5% biomethane recovery and 99.5% CO2e emission reduction, not estimated ranges. The system removes H2S to below 4 ppm and reduces oxygen content to under 10 ppm in your final product. The modular design scales from 30 Nm³/hour for small farm digesters up to 1,300+ Nm³/hour configurations for industrial facilities. Each unit arrives factory-tested and ready for hookup to your existing infrastructure.
"99pt5 stands out by guaranteeing 99.5% biomethane recovery and 99.5% CO2e emission reduction, coupled with the lowest operating expenses."
Ideal projects and gas qualities
The BioTreater handles feedstocks with variable H2S concentrations from agricultural waste, food processing residuals, and municipal wastewater treatment plants. System providers integrate these units into projects where clients demand guaranteed performance, low maintenance schedules, and remote monitoring capability through smartphone apps.
Costs, footprint, and deployment
Installation typically completes within 12 to 16 weeks from order. The compact footprint and skid-mounted design mean you need less site preparation than traditional processing trains.
Integration tips for system providers
Connect the unit between your digester outlet and final gas compression or injection point. The BioView software platform gives your operations team real-time monitoring and diagnostic data without requiring on-site technical staff.
2. Amine gas sweetening plants
Amine gas sweetening uses liquid chemical solvents (typically monoethanolamine, diethanolamine, or methyldiethanolamine) to absorb hydrogen sulfide and carbon dioxide from gas streams through a reversible chemical reaction. You run your contaminated gas through an absorption tower where it contacts the amine solution, then regenerate the rich amine in a separate stripper column using heat. This regenerative process makes amine plants one of the most established h2s removal technologies for large-scale gas processing.
Core process and flow scheme
Your gas enters the bottom of the absorber column and flows upward through structured packing while lean amine solution cascades down from the top. The H2S transfers into the liquid phase and forms a weak chemical bond with the amine molecules. The rich amine then moves to a regeneration tower where you apply heat (typically 240-260°F) to reverse the reaction and release the acid gases. You cool the lean amine and recycle it back to the absorber, creating a continuous loop that requires only makeup amine to replace small losses.
Suitable gas compositions and scales
Amine systems handle inlet H2S concentrations from 100 ppm to several percent by volume and work effectively across gas flow rates from 1 million to 500+ million standard cubic feet per day. You need sufficient pressure (typically 400+ psi) to drive efficient mass transfer in the absorber. These plants process natural gas, associated gas, and biogas when your project scale justifies the capital investment and you have access to reliable utilities.
"The regenerative process makes amine plants one of the most established technologies for large-scale gas processing."
Typical performance and emission control
You achieve H2S removal down to 4 ppm or lower depending on your amine formulation and operating parameters. The stripped acid gas requires additional treatment through thermal oxidation, Claus units, or reinjection wells to meet emission regulations. Amine degradation products and carryover into your product gas demand careful monitoring and filtration.
Capex, Opex, and utility demands
Expect capital costs from $2-10 million for small to mid-scale units, with larger facilities running significantly higher. Your operating expenses include natural gas or steam for regeneration (substantial energy consumer), amine makeup, corrosion inhibitors, and filtration media. You need reliable power, cooling water, and fuel gas supplies to maintain continuous operation.
When amines make sense for biogas
Choose amine systems when you process high-volume biogas streams (500+ scfm) with elevated H2S content and require simultaneous CO2 removal for pipeline injection. The technology suits centralized biogas upgrading facilities serving multiple digesters or landfills where economies of scale offset the higher complexity and utility requirements compared to simpler desulfurization methods.
3. Liquid redox desulfurization
Liquid redox systems convert hydrogen sulfide into elemental sulfur through a catalytic liquid process that uses chelated iron compounds as the active agent. Your contaminated gas contacts an iron solution in an absorber vessel where the H2S oxidizes into solid sulfur particles. The spent solution moves to a separate oxidation tank where air regenerates the iron catalyst back to its active form, creating a closed loop that continuously recycles the chemical without consuming it.
Chemistry and process configuration
The process runs on two linked reactions that work in sequence. First, the ferric iron (Fe³⁺) in your absorber solution reacts with H2S to produce solid sulfur and ferrous iron (Fe²⁺). You then pump this iron-depleted solution into an oxidizer vessel where injected air converts the ferrous iron back to ferric iron. The chelating agents (typically EDTA or similar compounds) keep the iron dissolved and active across pH ranges from 7 to 9. You filter out the produced sulfur through pressure or vacuum filtration and sell it as a commercial product while the regenerated solution returns to your absorber.
Operating window and gas limits
Your inlet gas should contain 500 to 10,000 ppm H2S for economical operation, though systems handle concentrations up to several percent. The technology works across gas flow rates from 50 to 5,000+ scfm and operates at pressures between 5 and 100 psig. You need gas temperatures between 50°F and 120°F for optimal catalyst activity and sulfur formation.
Efficiency, sulfur handling, and byproducts
You remove over 99.9% of inlet H2S when you design and operate the system correctly. The sulfur byproduct exits as a wet cake containing 50-70% elemental sulfur that you can sell to fertilizer manufacturers or sulfuric acid producers. Minor side reactions produce thiosulfates and sulfates that accumulate in your solution and require periodic purging.
"The chelating agents keep the iron dissolved and active across pH ranges from 7 to 9."
Cost profile and plant complexity
Capital costs run $500,000 to $3 million depending on gas volume and H2S loading. Your operating expenses include electricity for air blowers, chelant makeup, filtration media, and sulfur disposal or sales logistics. These systems require 316L stainless steel construction to resist corrosion from the iron solution and acid gas.
Pros, cons, and when to choose it
Liquid redox offers minimal water consumption and produces a saleable sulfur product that offsets operating costs. You avoid the chemical waste streams typical of non-regenerative h2s removal technologies. However, the higher capital cost and control sophistication make it suitable primarily for mid to large biogas facilities (200+ scfm) with consistent H2S concentrations above 1,000 ppm where sulfur sales revenue justifies the investment.
4. Chemical scrubbing and oxidation
Chemical scrubbing systems inject your gas stream into liquid absorbent towers where sodium hydroxide (caustic soda) neutralizes the H2S through direct chemical reaction. You then oxidize the absorbed sulfide compounds using hydrogen peroxide or sodium hypochlorite to convert them into soluble sulfate salts that leave the system through a continuous blowdown stream. This approach ranks among the simplest h2s removal technologies to operate when you need compact equipment and proven performance across varying gas qualities.
How caustic and oxidizing scrubbers work
Your contaminated gas flows upward through packed-bed columns while the caustic solution sprays down from the top, creating countercurrent contact that maximizes absorption efficiency. The caustic first neutralizes the H2S into sodium sulfide, then the oxidizing agent converts this intermediate compound into sodium sulfate in a secondary reaction tank. You control the process by monitoring pH levels (typically 8 to 10) and adjusting chemical feed rates to match your inlet H2S concentration and gas flow.
Design choices and performance ranges
Vertical packed towers using fiber-reinforced plastic (FRP) construction resist the corrosive environment and minimize capital costs. You achieve over 99.9% H2S removal from inlet concentrations between 100 ppm and 5,000 ppm across gas flows from 50 to 2,000 scfm. The systems handle wide fluctuations in gas composition without performance degradation, giving you operational flexibility when your digester output varies.
"The systems handle wide fluctuations in gas composition without performance degradation."
Operating costs, waste streams, and safety
Chemical costs dominate your operating budget at $0.50 to $2.00 per pound of H2S removed, depending on local pricing and oxidant choice. The blowdown stream requires treatment or disposal as a high-pH, high-salt waste that cannot discharge directly to surface waters. Caustic handling demands proper PPE and spill containment infrastructure at your facility.
Best fit applications in biogas and gas
Choose chemical scrubbing when you process biogas from landfills or food waste digesters where H2S concentrations fluctuate daily and you need a system that operators can manage without specialized technical training. The compact footprint suits retrofit projects where existing facilities add upgrading capacity within limited space.
5. Biological H2S removal systems
Biological H2S removal uses living microorganisms (primarily sulfur-oxidizing bacteria) to convert hydrogen sulfide into elemental sulfur or sulfate compounds through natural metabolic processes. You introduce your contaminated gas into a reactor vessel containing specialized bacteria that consume the H2S as their energy source while using oxygen from controlled air injection. This biological approach represents one of the most environmentally friendly h2s removal technologies because it operates at ambient temperature, requires minimal chemical inputs, and produces non-hazardous byproducts.
Types of biological systems
Biotrickling filters pass your gas through a packed bed colonized with sulfur-oxidizing bacteria while continuously recirculating a liquid nutrient solution that keeps the microbes active. Bioscrubbers first absorb H2S into a liquid phase in a scrubber tower, then transfer the sulfide-rich solution to a separate bioreactor where bacteria oxidize the absorbed compounds. Both configurations handle inlet concentrations from 100 to 5,000 ppm across flow rates between 50 and 1,500 scfm.
Treatment performance and stability
You achieve 90 to 99% H2S removal when you maintain optimal conditions for bacterial growth: pH between 6.5 and 8.0, temperatures from 60°F to 95°F, and consistent moisture levels. The systems need several weeks to establish stable bacterial populations during startup, and performance drops quickly if your gas stream dries out or temperatures fall outside the viable range for extended periods.
"This biological approach represents one of the most environmentally friendly technologies because it operates at ambient temperature and requires minimal chemical inputs."
Costs, footprint, and maintenance
Capital costs range $150,000 to $800,000 depending on your gas volume and required removal efficiency. Operating expenses stay low at $0.10 to $0.40 per pound of H2S removed since you only pay for electricity, nutrients, and periodic media replacement. You need larger vessels than chemical systems to provide sufficient residence time for biological reactions.
Where biology outperforms chemistry
Choose biological systems when you process biogas with stable H2S concentrations below 2,000 ppm and can provide consistent operating conditions. The low chemical consumption and minimal waste generation make biological methods ideal for agricultural digesters and wastewater treatment plants where sustainability matters more than rapid installation or operation in extreme climates.
6. Non regenerative H2S scavengers
Non-regenerative scavengers use chemical compounds that irreversibly bind hydrogen sulfide into stable reaction products that remain in your system or exit through blowdown streams. You inject these scavengers directly into your gas flow or circulate them through contact vessels where the H2S reacts and converts to non-volatile compounds. Unlike regenerative h2s removal technologies, you consume the scavenger continuously and dispose of the spent product, making this approach most economical for intermittent treatment or low H2S loads.
Liquid and solid scavenger types
Triazine-based liquid scavengers dominate the market for gas treating applications, reacting quickly with H2S to form dithiazine compounds that stay dissolved in hydrocarbon liquids. Solid scavengers (iron oxide, iron sponge, zinc oxide) physically contact your gas stream in packed beds and form metal sulfide compounds that saturate the media. You replace solid media when sulfur loading reaches 30 to 40% by weight of the bed capacity.
Reaction mechanisms and capacity
Triazine scavengers achieve stoichiometric ratios of 2 to 3 moles H2S per mole of active ingredient under ideal conditions. Iron-based solids remove 10 to 20 pounds of H2S per 100 pounds of media before requiring disposal.
Cost per unit of sulfur removed
Your chemical costs run $2 to $8 per pound of H2S removed depending on scavenger type and dosing efficiency. Spent product disposal adds $50 to $200 per ton when you account for transportation and regulatory requirements.
Operating constraints and risks
Scavenger systems need gas humidity above 30% for effective liquid distribution and require precise injection control to avoid over-treating. Byproduct accumulation in pipelines creates fouling problems that demand periodic pigging or cleaning.
Best use cases for scavenger packages
Choose scavengers for wellhead treating, pipeline protection during startup, and low-volume gas streams (under 100 scfm) where capital investment in permanent equipment cannot be justified. The technology suits temporary operations and remote locations without power or utilities.
7. Fixed bed adsorption and carbon
Fixed bed adsorption systems pass your contaminated gas through vessels packed with solid materials that physically trap hydrogen sulfide molecules on their surface through van der Waals forces and chemical bonding. You operate these beds at ambient temperature and moderate pressure until the adsorbent reaches saturation, then either regenerate the media or replace it with fresh material. This technology offers one of the simplest h2s removal technologies to implement when you need low outlet concentrations without complex controls or chemical handling.
Adsorbent types and mechanisms
Activated carbon dominates fixed bed applications because its high surface area (800 to 1,500 m²/g) provides exceptional capacity for H2S adsorption. The carbon surface oxidizes hydrogen sulfide into elemental sulfur that remains bound to the pores. Impregnated carbons containing potassium hydroxide or potassium carbonate boost capacity by converting H2S into potassium sulfide compounds. You achieve inlet concentrations from 100 to 5,000 ppm with outlet levels below 1 ppm when you size beds correctly.
Design, sizing, and breakthrough
Your vessels need sufficient bed depth (typically 3 to 6 feet) to provide contact time between 2 and 10 seconds. You calculate required media volume by dividing your expected sulfur loading by the adsorbent's rated capacity (10 to 30% by weight for activated carbon). Breakthrough occurs when your outlet H2S concentration rises above specifications, signaling media exhaustion.
"This technology offers one of the simplest methods to implement when you need low outlet concentrations without complex controls or chemical handling."
Operating costs and replacement cycles
You spend $1.50 to $4.00 per pound of H2S removed when you account for media costs and disposal fees. Replacement intervals range from 2 weeks to 6 months depending on inlet concentration and gas humidity. High moisture content accelerates bed exhaustion.
When to use adsorption in a project
Choose fixed bed systems when you need final polishing after primary treatment or process low-volume gas streams (under 200 scfm) requiring outlet H2S below 4 ppm. The technology suits remote installations without power and provides simple backup protection during maintenance on regenerative equipment.
Bringing it all together
Your choice among h2s removal technologies depends on gas volume, H2S concentration, and project economics. Amine systems and liquid redox fit large facilities with steady high-sulfur loads, while biological methods suit stable low-concentration streams where sustainability matters. Chemical scrubbers handle fluctuating conditions in mid-scale operations, and scavengers or adsorption beds work best for intermittent treatment or final polishing.
Project success requires matching the technology to your specific feedstock and operational constraints. You reduce risks by choosing vendors who guarantee performance metrics, provide integrated automation, and support remote monitoring. The difference between systems that deliver predictable returns and those that drain budgets shows up in operating costs, maintenance schedules, and actual removal efficiency under field conditions.
Discover how 99pt5's integrated BioTreater system delivers guaranteed 99.5% biomethane recovery with the lowest operating expenses in the industry.